how long does it work and how long does it actually last?
How long a CO2 scrubber lasts during a dive depends on many factors. I will mention some of the ways that CO2 can be removed from the breathing gas. I will also discuss ways to measure how long a scrubber will last (called the endurance time) and some of the factors that change it, but first will discuss why it is necessary at all.
A: Where is the CO2 coming from and why is it necessary to remove CO2?
People produce CO2 just by being alive. The amount of CO2 produced depends on the person’s work load (exercise level). At rest it may be as low as 0.3 to 0.5 L/min and for moderate work it may be 1.5 L/min. At hard work it may be about 4 L/min. These gas volumes are measured at Standard Temperature and Pressure, Dry (STPD, i.e. 0°C and at 1 atm). They do not change with depth.
The body tries to maintain a fairly constant level of CO2. In fact, CO2 is the primary signal used by the body to control how much a person breathes (the minute ventilation). Similarly to the pO2 in your rebreather, it is the partial pressure of CO2 (pCO2) that matters, not the percentage (fraction) of CO2. The average expired pCO2 for a diver varies from some 2.5 to 4 kPa (25 to 40 mbar). The exact value varies with workload, how difficult it is to breathe and how much CO2 that is in the inspired gas.
B: Ways to remove CO2
The common way to remove CO2 from a diver’s rebreather is by chemical absorption (“scrubbing”). Most modern commercial absorbents consist of a mixture of calcium hydroxide and sodium hydroxide. Lithium hydroxide is sometimes used in cold applications. The materials have CO2 absorption capacities and the temperature influences them differently.
Other methods for removing CO2 from gas are sometimes used in submarines. It may be possible to liquefy the gas by cooling it and then separate its components. However, these two methods are both energy intense and are not applicable to diving.
Superoxides are used in some rebreathers. They work by absorbing CO2 and releasing O2. However, they tend to react violently with water.
C: How do we determine the endurance time of a CO2 scrubber?
We should use divers as test divers and simply measure the pCO2 in the gas leaving the scrubber. However, there are many reasons why this is not a good idea. Some rebreathers last many, many hours even at great depths in cold water. The exposure to the cold water and the decompression times involved with deep diving make tests with divers prohibitive. Also, many divers would find it hard to maintain some of the high work rates needed for testing. People also vary in their CO2 production so it would not be easy to get consistent numbers. It would also be extremely boring. We need to measure the scrubber endurance time consistently at depth, for great lengths of time and in any water temperature. Hence, we use a breathing simulator (sometimes called a breathing machine) to determine the scrubber endurance.
The simplest form of breathing machine is just a piston moving back and forth in a cylinder. However, to determine the scrubber endurance time, a more sophisticated breathing simulator has to be used (see Figure 1). Its function is to “breathe like a diver” in most respects. In fact, a breathing machine should mimic a diver so well that a rebreather should not be able to tell the difference between it and a diver (except that the breathing simulator breathes more regularly). To do this a breathing simulator must exhale warm, moist air with the correct CO2 content. It must take breaths of the same size range as a diver and should breathe with the same range breathing frequencies.
During testing, the rebreather must be immersed in water that is kept at the desired temperature and it must be held at the desired depth (usually in a hyperbaric chamber). Scrubber endurance testing is not something that divers can undertake by themselves. Not all manufacturers of rebreathers can either, so they have tests done at a separate test facility.
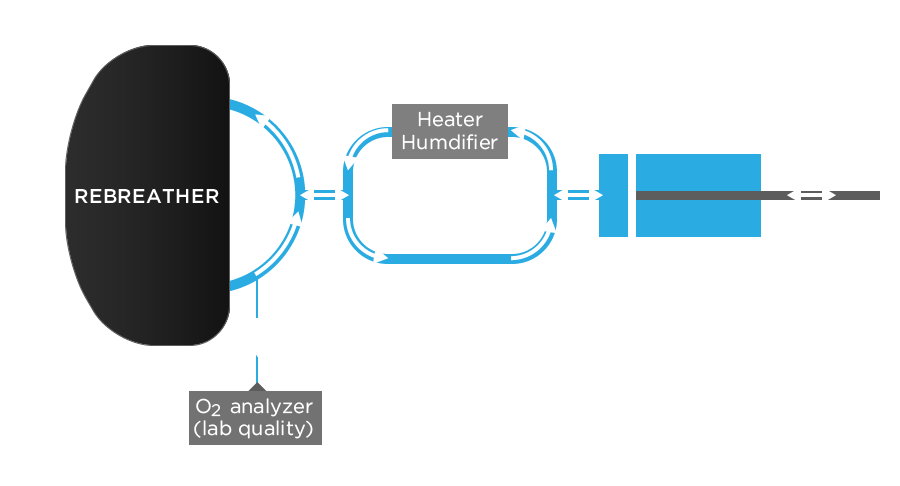
Figure 1. A schematic drawing of a breathing simulator used to determine the endurance time of a rebreather’s scrubber. Arrows indicate the direction of gas. For clarity, valves directing the flow are not shown, nor are the water bath and the hyperbaric chamber.
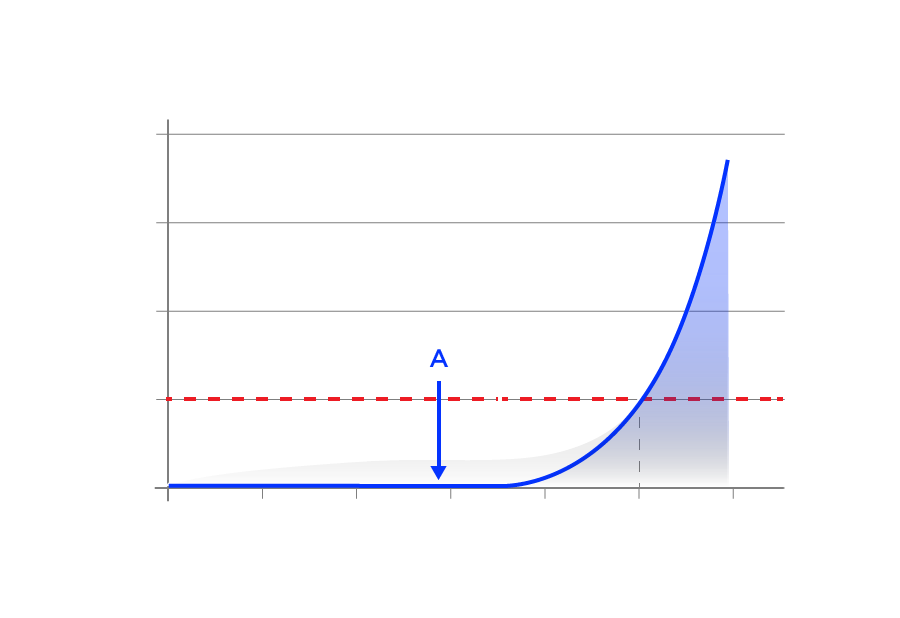
During a scrubber endurance test, the gas leaving the scrubber (i.e. the gas about to be inhaled) is analyzed for its pCO2. The easiest way to judge endurance time is to graph how the pCO2 varies with time. An example is shown in Figure 2, line A. At the beginning of the test, the pCO2 stays at essentially zero, i.e. all CO2 is absorbed. For most scrubbers it stays at zero until 60 to 80% of the absorbent is used up. If the absorbent is very cold there may be a slight bump of CO2 for several minutes until the absorbent warms up. In Figure 2 some CO2 starts to get through the scrubber after about 70% of the endurance time. (Endurance time here is defined as time to pCO2=0.5 kPa, this choice is discussed below.) Note how quickly the CO2 climbs after it becomes measurable. It reaches 1 kPa (10 mbar) at about 110% of the endurance time and doubles again (2 kPa, 20 mbar) at 118% of the endurance time. Things go badly very quickly.
Line B in Figure 2 shows a pattern that is different than line A. Here, some CO2 appears almost immediately. The pCO2 stays slightly elevated throughout the test. Towards the end the CO2 climbs as fast as line A. This type of pattern is typically seen in small scrubbers, in bigger ones that are used in the cold or used at higher work rates than intended. When the absorbent can’t quite keep up the pCO2 may stay at an almost constant level or have a slow increase. This pattern can also be seen in badly packed scrubbers where some CO2 can channel past the absorbent, hence the term “channeling”.

Figure 2. Plot of how the pCO2 in the gas leaving the scrubber changed during tests of different scrubbers. The red, interrupted line shows the cut-off limit for pCO2 at 0.5 kPa, 0.5%SEV (surface equivalent value), 5 mbar. See text for details.
Choice of cut-off for pCO2
Almost all standards for testing of scrubber endurance, for instance the European rebreather standard EN 14143, the U.S. Navy, the U.S. National Institute of Occupational Safety and Health (NIOSH) agree that the cut-off level for pCO2 should be 0.5 kPa. There are two reasons:
1) how people react to inhaled CO2,
2) the rapid rise in pCO2 leaving the scrubber after it reaches this level.
1) The response to CO2 in the inhaled gas varies from person to person. Some people are quite sensitive to it and increase their minute ventilation, while others don’t seem to react at all. We know that inspired CO2 increases the risk for O2 toxicity and that it has narcotic properties at elevated levels. The breathing resistance that is present in a breathing apparatus makes the influence of inspired CO2 worse. Levels of pCO2 above 1 kPa are best avoided. Mention: For a further discussion, see “insert its title” on Shearwater’s web site discusses this in more
At least one manufacturer has claimed in its newsletter that their rebreather was so easy to breathe that they gave the scrubber endurance time to 2 kPa (20 mbar). Such statements reflect a great ignorance of reality, would put divers at great risk, and must not be trusted.
2) As has been pointed out above, there is a drastic change in pCO2 in the gas leaving the scrubber towards the end of its life. Put differently, a small change in the scrubber’s performance will have a big impact on the CO2 levels. Therefore, the cut-off level has to be kept low so that a diver stays away from inhaling gas with a high pCO2 during an actual dive.
Magnitude of changes in the scrubber endurance time during normal use
Typically, a rebreather has a stated scrubber endurance time, most likely determined according to the European Diving Standard EN 14143. EN 14143 endurance times are determined at a water temperature of 4 °C (39 °F). As an example, say that the endurance was listed as 2 hours. Is the endurance always 2 hours? The answer to that is “no”. In fact, it is rarely 2 hours. Because of the complexities of CO2 scrubbing and the many ways that a rebreather can be used, the stated endurance time has to be a low value.
The endurance can vary greatly even for the same scrubber, packed the same way, by the same person. The changes described below are based on actual measurements from a number of rebreathers. Since the rebreathers vary very much in design and capacity, the magnitude of changes has to be general and should be taken with caution. The purpose of the description is really only to show the possible variations and to illustrate that there is no hard and fast rule.
Workload. How hard you work during a dive has the biggest effect on scrubber endurance: you may be waiting quietly for a fish to be visible for a photo or swimming very hard upstream to get to the boat. Your breathing may vary from 10 to 15 L/min to over 75 L/min. The CO2 load that the scrubber has to manage will change with it. A 5-fold increase in work rate will not just reduce the scrubber endurance time by a factor of 5, but may actually decrease it by a factor of 10.
Water temperature. The water temperature is another big factor. If you are diving in warm water, the absorbent will be warmer than it would be in cold water. Generally the absorbent is better at removing the CO2 when it is warm. For instance, diving in 15 °C (59 °F) water may increase the endurance by 50% compared to the 4 °C water where the stated endurance was most likely determined. Diving in 30 °C (86 °F) water may increase the endurance by another 25 to 40%.
Diving depth. The depth has an effect too. An increasing depth tends to decrease the scrubber endurance. However, the size of this decrease is hard to specify because it depends on the rebreather design and type of absorbent used. The likely reason for this decreased endurance is that increasing depth means denser gas which is better at cooling the absorbent.
Packing variation. Even if the same person packs the same scrubber with absorbent from the same container the endurance may vary by 5 to 10% from one time to another.
Diver’s diet. The amount of CO2 produced by the diver varies by the energy source used by the body. Fat yields 0.7 L of CO2 for every L of O2 consumed. Protein yields about 0.8 L, while carbohydrates yield 1 L of CO2. If a diver works hard enough to produce lactic acid in the muscles, then the CO2 production will be well over 1 L for every L of O2. So, the CO2 production may vary by ±15%.
Combined effects on the scrubber endurance time. With all these factors influencing the endurance time, the 2-hour scrubber may actually last anywhere from one hour to eight or even 10 hours.
Why does the scrubber endurance vary so much?
Much of the variation in the scrubber endurance times can be summarized in one term: efficiency. If CO2 absorbent is given plenty of time under ideal conditions it will absorb a known amount of CO2. A technical term for this is “theoretical capacity” and one can think of it as 100% capacity. The efficiency is the fraction of the theoretical capacity that is actually used. For example, if all the theoretical capacity is used then the efficiency will be 100%, while if only half is used the efficiency is 50%.
During real use the efficiency will be less than 100%. During very gentle use (quiet breathing in warm water) the efficiency may be 60 to 80%. During challenging use (hard work in cold water) the efficiency may be less than 10%. Bigger scrubbers or multi-scrubber rebreathers tend to have higher efficiencies.
The theoretical capacity cannot be used to determine the scrubber endurance time when the absorbent is in a scrubber.
What influences the efficiency of a scrubber?
Several factors determine how much CO2 a scrubber will remove. Some factors have been mentioned above but are included for completeness.
- Absorbent composition. A manufacturer of absorbent can choose which chemical components that go into a certain absorbent to tailor it for good performance for a particular use. An absorbent intended for a rebreather (where the flow is pulsatile and with a fairly high CO2 concentration) might be different from one intended for CO2 removal in a hyperbaric chamber (a constant flow of gas with a fairly low CO2 concentration). A medical grade absorbent (used in a patient’s respirator) can be designed for low workloads at room temperature and would then not be suitable for diving. Lithium hydroxide can be included in absorbents used in cold environments. Diving, with its very large range of workloads, temperatures and gas densities, is one of the most difficult uses.
- Absorbent packaging. Most absorbent is sold in a granular form. The granule size is generally rated on a scale from 4 to 12, where 4 is a large granule and 12 is a small one. Often the absorbent comes in a range of sizes, for instance 4-8 or 8-12. Some pre-packaged canisters have other numbers. One manufacturer sells a product that consists of powdered absorbent mixed with a polymer, shaped with defined ridges and then rolled up.
Generally, smaller granules have more surface area and tend to be more efficient than the larger granules, but increase the breathing resistance. A rebreather manufacturer has to decide on the trade-off between scrubber efficiency and breathing resistance when deciding which absorbents may be used in a particular rebreather. There is a similar trade-off when deciding on the shape of the ridges of the rolled-up type of absorbent.
- In general, chemical reactions are slow at low temperatures. This means that the practical performance of CO2 absorbents is worse during diving in cold rather than warm water. Diving in very warm water may also reduce the efficiency of the chemical reactions.
- User work rate / minute ventilation. The minute ventilation (the volume of gas expired) of a diver can vary widely. A high minute ventilation gives the absorbent less time to react with CO2, resulting in a lower efficiency compared to lower work rates.
-
Scrubber size. A larger scrubber tends to be more efficient than a small one because it will hold more of a breath. This gives the absorption more time. A technical term used to describe this is dwell time, i.e. a larger scrubber has a longer dwell time than a small one.

Figure 3. A schematic illustration of four types of scrubbers with different shapes and flow directions. The arrows indicate the direction of gas flow.
- Scrubber design. There are many types and shapes of scrubbers and they generate different flow patterns (Figure 3). In scrubber A the flow of gas is along the axis of the scrubber (hence “axial flow”). Scrubber B has axial flow as well, but the scrubber is longer and narrower than A. In scrubbers C and D the flow is from the outside to inside or from inside to outside of a circular scrubber (“radial flow”). Some scrubbers have a cross section that is cylindrical while some have an oval one. Some rebreathers have more than one scrubber. The exact design chosen by a rebreather manufacturer is often determined by the available space in a rebreather. Some manufacturers design the rebreather such that the scrubber is not in direct contact with the water. That way it will likely stay warmer and last longer.
- Gas density / depth. With increasing depth, the gas passing through the scrubber gets denser and therefore cools the absorbent more, making it less efficient. At very great depths the rate of gas diffusion decreases. Simply put, other gas molecules are in the way of the CO2 on its way to the absorbent.
- Moisture content. The chemical reactions in the absorbent require some moisture or water vapour to work well. Typically, a range of 15 to 20% is best.
Why is only one endurance time given for a scrubber?
Given the widely varying efficiencies of the CO2 scrubber is essentially impossible to determine what the scrubber endurance might be. For instance, some dives will be deep, then followed by decompression at shallower and shallower depths and finished by a strenuous swim upstream – all at varying water temperatures. It is not possible to predict what the scrubber endurance might be. Therefore, one standardized condition is chosen. For safety, one is chosen where the efficiency is expected to be low – a worst case style of testing.
Before changing the absorbent, some divers will use their rebreathers much longer than the stated scrubber endurance. We have seen why that is possible. However, this can be a very tempting, but dangerous, way of diving because the safety margins are gone. When the scrubber is nearing the end of its endurance it is not capable of challenging use. Figure 4 illustrates a test where the workload was changed from low to moderate and back throughout the test. In the early part of the test the pCO2 remained low. At around 50% of the test, some CO2 started to appear, but only when the workload was moderate. When the workload dropped to low again the scrubber was able to remove more of the CO2 (pCO2 dropped). At about 90% of the test, the pCO2 almost broke through the cut-off limit, but dropped as the workload dropped. At about 110% there was a big increase in pCO2 as the workload increased and the pCO2 increased from about 0.6 kPa to about 1.6 kPa in a very short time. What may have seemed to be a safe pCO2 at the low work rate rapidly tripled when the work load was increased. This example illustrates the danger of overusing the absorbent. An almost used up scrubber can’t handle a challenge.

Figure 4. Plot of how the pCO2 in the gas leaving the scrubber changed during a test where the workload varied. See text for details.
Rebreather manufacturers may size the scrubber so that it should last longer than the gas supply. The idea is that one must run out of something one can measure before one runs out of something that one can’t measure. This might work for most dives, but might not if the diver stresses the scrubber more than the designer intended.
Existing standardized ways to measure scrubber endurance times
As mentioned earlier, for civilian rebreather diving, the European standard EN 14143 specifies how a scrubber’s endurance time must be tested. The entire rebreather is submerged in water at a temperature of 4 °C. A breathing simulator is set at a minute ventilation of 40 L/min with a CO2 production of 1.6 L/min. Tests are run at depths determined by the gas mixture used (nitrox, heliox, trimix or 100% O2). As a sort of stress test, one more type test is run: after half the previously determined endurance time the simulated work rate is almost doubled for five minutes. The pCO2 must remain below 5 mbar (0.5 kPa). All tests are repeated three times.
Recently, the British Standards Institute published a standard for tests of CO2 absorbent to be used in diving and other conditions (BS 8618). A glass tube in which CO2 rich gas flows continuously is packed with absorbent. Endurance is measured only at room temperature and at atmospheric pressure (not diving depths). Different absorbents are graded and it is supposed that different absorbents with the same grade are equivalent. However, some absorbents do not work well at low temperatures, something that may not be apparent from tests at room temperature. Therefore, comparisons of different absorbents intended for diving using this BS 8618 standard must be questioned. Use only the absorbent(s) recommended by the rebreather manufacturer.
Summary
The scrubber endurance time varies greatly due to many factors, primarily the diver’s workload and the water temperature. Therefore, the scrubber endurance time is given during conditions where the absorbent efficiency is expected to be low, i.e. at a moderate workload, in cold water and at the maximum depth that the rebreather will be approved for. Use only the absorbent that the rebreather manufacturer has recommended.
---

Written by Dan Warkander
Dan Warkander is an Engineer & Respiratory Physiologist who has worked with divers and their breathing equipment for over 30 years: air dives to 57 msw (190 fsw), Heliox dives to 450 msw (1500 fsw) and hydrogen-oxygen (hydrox) dives at 120 msw (400 fsw). He has led over 1,000 experimental dives; found and implemented breathing resistance limits for diving and dry-land use; developed a simple to use CO2 scrubber gauge.